The Science of Carryover Cooking: What Happens After You Cook
If you pay attention, you’ll notice that in most of our blog posts about cooking meat, all the roasts, the whole chickens, the chops, and (almost) all the steaks are pulled at temperatures below the final doneness temperatures that we recommend. The reason we typically give for this is something called “carryover cooking.”
It’s easy enough to say that foods will continue to cook after being removed from the oven or smoker and to make allowance for it. But, here at ThermoWorks, we believe that a deeper understanding of the thermal and physical forces involved in cooking will actually lead to more intuitive cooking and, yes, even better results. So, this week,
The physical basis of carryover cooking
There are a few basic physical laws that govern carryover cooking in foods. And, although they can seem a little daunting, the basic ideas aren’t difficult to grasp.
The second law of thermodynamics
As you may have learned in middle school, the second law of thermodynamics tells us that the degree of disorder in a system will increase unless work is performed on it. Which is to say that unless you plug something in like a fan or refrigerator, heat can only flow “downhill.”
For example, if you place a cold drink on a hot windowsill on a summer’s day, the drink will not somehow get colder, surrendering its meager heat to further heat the windowsill. Anyone knows that the drink will heat up. And if, after a few minutes, you pick the glass back up, the spot where it had been sitting will have cooled down—having surrendered some of its heat to warm the drink.
What on earth does this have to do with carryover cooking? A lot, actually. It means that the hot outside of a roast will share its heat with the colder inside, and it will do so with absolute physical certainty. This is the central principle of carryover cooking. The other principles we mention hereafter are all secondary to it. This tendency of heat to be shared until an equilibrium is reached is exactly what causes carryover cooking, and understanding it on that
Heat capacity
The next concept to understand in carryover cooking is heat capacity. Heat capacity is the ability of a certain substance to store thermal energy. And, by extension, heat capacity
Another thought experiment: Imagine, if you will, a piece of aluminum foil inside of a 350°F (177°C) oven. Without much fear of repercussion, you might reach into that oven and pull out that sheet or wad of foil. You’ve probably grabbed foil from an oven before. However, if even a single drop of 350°F (177°C) oil were to splash on your hand while you were deep-frying something, you would scream in pain!
What gives? Weren’t the foil and the oil at the same temperature? Well, yes, in fact, they were, but oil carries more thermal energy per degree of temperature than does aluminum. Depending on the type, oil can carry as much as 2.2 times the thermal energy of aluminum at the same temperature. And water carries even more! Leaving the units off for the sake of understanding the raw numbers, aluminum has a heat capacity of 0.91, olive oil has 1.97, and water has 4.19! That’s why you can heat a pot of oil to 350°F (177°C) in roughly the same amount of time it takes to heat a pot of water to boiling, 212°F (100°C).
What those heat capacity measurements represent in physical terms is the amount of energy (measured in Joules) needed to heat one kilogram of each substance one degree Kelvin (1.8°F).* So one kilogram of water takes 4.19 joules to raise its temperature 1°C (1.8°F), but aluminum only takes 0.91 joules for the same temperature change.
*These heat capacities are based on mass. Volumetric heat capacities can also be derived that measure the energy required to change a certain volume of a substance by 1°C. Water still has a much higher specific heat capacity in that case.
Heat transfer formula (Heat equation)
The way that heat distributes itself through a substance is, as noted above, unidirectional. But the movement can be more accurately described by the heat equation.
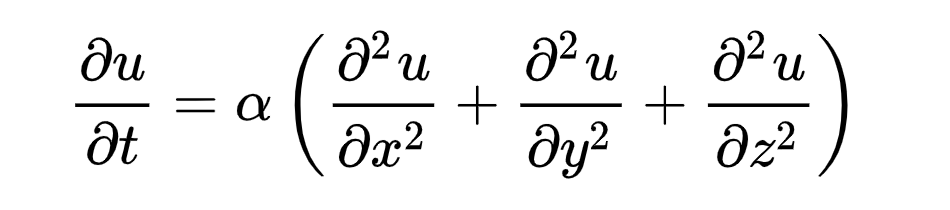
The heat equation is a handy (if admittedly slightly intimidating) partial differential equation that describes heat moving over time. What it means is not hard to grasp, even if the mathematics themselves may be.

What the heat transfer formula means, physically
The heat equation says that heat is transferred directly across adjacent points…it doesn’t skip around. It also says that areas with a greater temperature difference will change more quickly—a 100°F plate stacked on top of a 40°F plate will heat the cooler plate faster than a 50°F plate stacked on top of it. That’s basically it: Adjacent heating, faster with a greater differential.
This is one of the most important physical principles in carryover cooking. It explains the heat flow inside a piece of steak, a roast, or even a loaf of bread. Heat is passed down the line from particle to particle, each bumping into the next and averaging out their energy, with more energy being pumped in from the oven/grill/pan each moment. This is what causes the phenomenon we call temperature gradients, the differences in temperature that exist between the hot surface of your food and the cooler thermal center. Temperature gradients explain why you see a grey band around a cross-section of improperly cooked steak: the outside was hotter than the inside and cooked faster.

The second law of thermodynamics says that the energy being pumped into the food can only flow to an area of lower energy, and heat capacity tells us how much energy is necessary to raise the temperature of the food by a certain amount, and the heat equation tells us how heat moves and where the temperature gradients come from.
Factors that affect carryover cooking
With that grounding in the physics of heat, we can look more closely at how heat operates in food while cooking. And one of the first things to think about in that regard is thermal mass.
Thermal mass
Thermal mass is roughly equivalent to total heat capacity—not the capacity per kilogram, but the capacity of the entire piece of food (or cookware) as a whole. (A New York strip steak has the same heat capacity per gram as a whole short loin, but nowhere near the total capacity of the whole because the short loin is bigger.) A whole loin has more thermal mass than a steak, and that means there’s more heat to go around when the loin is removed from the cooking environment. So the size of any given piece of food has a role to play in carryover cooking.
Geometry: the shape of the food
How the food is cut matters, too. A single steak has a lot of surface area, compared to its volume. That means there is a lot of space to vent heat into the air, cooling a larger portion of the meat much faster. Think of a steak compared to five steaks stacked together (like a loin). The amount of space available for heat to escape is proportionally much smaller for the loin.

Ultimately, a sphere is the shape most prone to carryover cooking (i.e. least prone to cooling) of all, because it has the least surface area for a given volume. Interestingly, a stuffed turkey is roughly spherical in shape. That’s why it’s a good reason to not stuff your turkey and to cook it to a slightly lower temperature!
Cooking temperature
Possibly the most straightforward factor for carryover cooking is the temperature of the cooking surface or oven itself. A high oven will pump more heat into any cut than a low oven will, and the extremes in temperature differential from the cooler center of the meat compared to the hotter surface will be much greater, causing more carryover cooking once you remove it from the oven.
Carryover cooking experiment
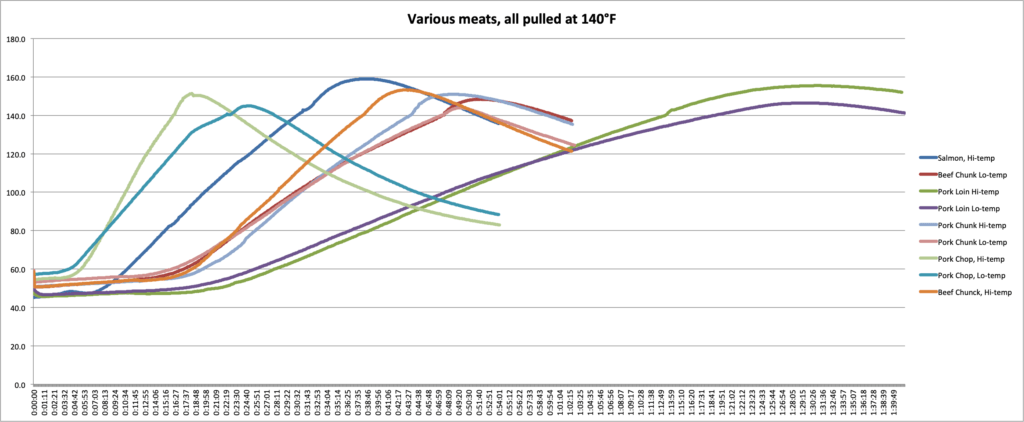
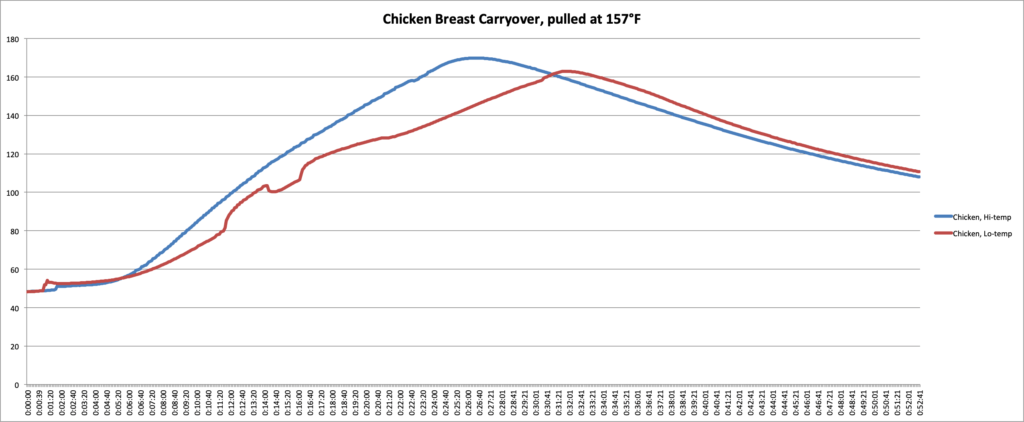
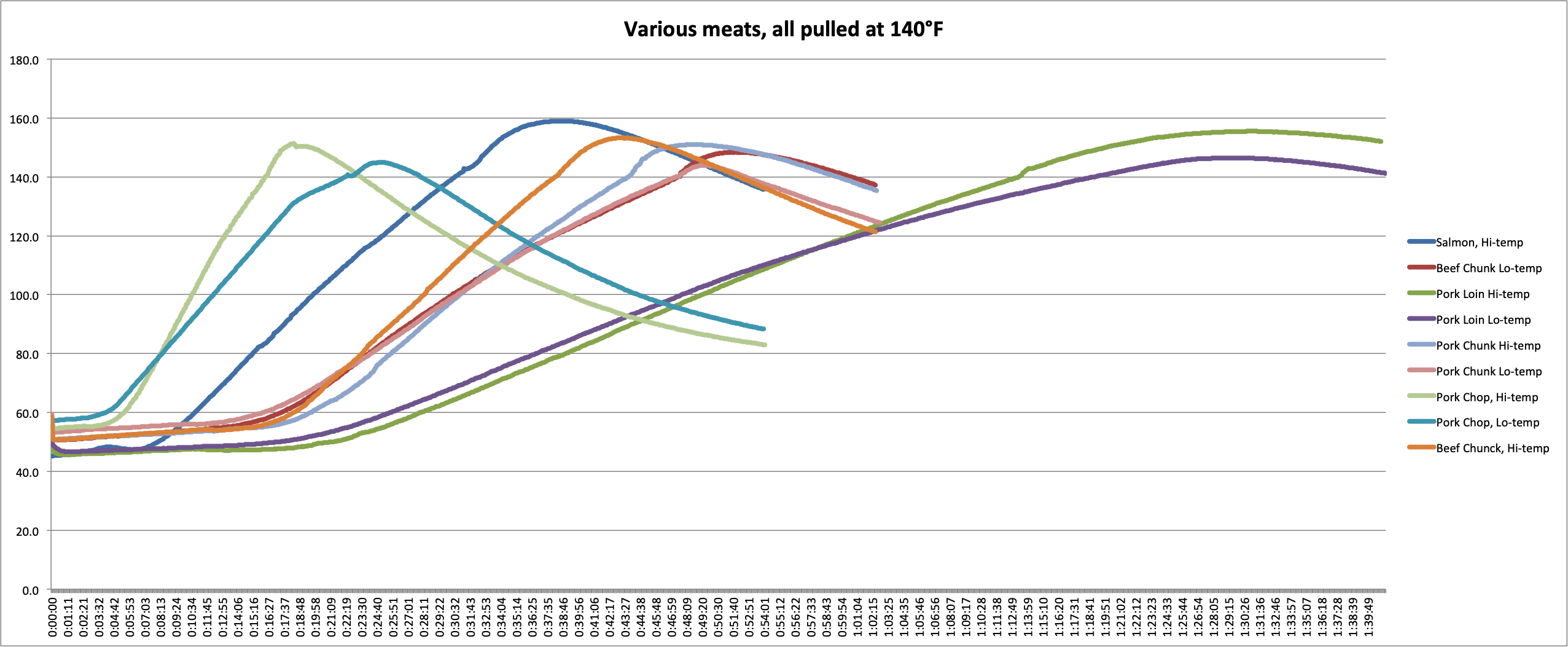
To demonstrate how carryover cooking is affected by mass, cooking temp, and the makeup of the food, we performed an experiment in our kitchen. We cooked two sets of meat, one in an oven set to 300°F (149°C), the other in an oven set to 425°F (218°C). Each set of meat consisted of a pork chop, a large chunk of pork and a large chunk of beef of comparable mass and shape to each other, a chicken breast, a fillet of salmon, and a section of pork loin.

We probed each piece

In the graphs and chart below, you can see the data we collected.
| Item | Mass | Pull temp | Highest temp achieved | Total carryover |
|---|---|---|---|---|
| Pork chop, cooked at 300°F | 150 g | 140°F | 144.9°F | 4.9°F |
| Pork chop, cooked at 425°F | 150 g | 140°F | 151.5°F | 11.5°F |
| Beef chunk, cooked at 300°F | 260 g | 140°F | 148.4°F | 8.4°F |
| Beef chunk, cooked at 425°F | 286 g | 140°F | 153.2°F | 13.2°F |
| Pork chunk, cooked at 300°F | 274 g | 140°F | 143.9°F | 3.9°F |
| Pork chunk, cooked at 425°F | 340 g | 140°F | 151.0°F | 11.0°F |
| Pork loin, cooked at 300°F | 1229 g | 140°F | 146.5°F | 6.5°F |
| Pork loin, cooked at 425°F | 1329 g | 140°F | 155.5°F | 15.5°F |
| Salmon, cooked at 300°F | 276 g | 140°F | 147.3°F | 7.3°F |
| Salmon, cooked at 425°F | 314 g | 140°F | 159.0°F | 19°F |
| Chicken, cooked at 300°F | 198 g | 157°F | 162.9°F | 7.9°F |
| Chicken, cooked at 425°F | 245 g | 157°F | 169.9°F | 12.9°F |
We can make several key observations by examining this data.
- First, we can see the effect that cooking temperature has. In every case, the meat that was cooked at the higher temp experienced more carryover than meats cooked at the lower temps. A pork loin cooked at 425°F (218°C) experienced 9°F (5°C) more carryover than a loin cooked at 300°F (149°C). If you want a pork loin that is not overcooked and you’re cooking at that higher temperature, you’ll need to pull it several degrees earlier!
- Second, we can see how mass affects the carryover. For the three pieces of pork cooked at each temperature, you can see the carryover increasing as mass increases. In the 300°F (149°C) oven, the chop had 4.9°F (2.7°C)of carryover, while the loin had 6.5°F (3.6°C). In the 425°F (218°C) oven, the carryover increased from 11.5°F (6.4°C) in the chop to 15.5°F (8.6°C) in the loin. (I don’t think the chop and the chunk were different enough to overcome experimental error in this experiment. Hence the chunk experiencing slightly lower carryover.)
- And third, we can see how the kind of meat affects the carryover. The beef chunks and the pork chunks had very similar masses and shapes but had very different carryover in each case. In the cooler oven, the beef carried over by 8.4°F (4.7°C) while the pork only carried over by 3.9°F (2.2°C). In the hotter oven, that gap remained: the beef had 13.2°F (7.3°C) of after-cooking carryover and pork had only 11.0°F (6.1°C).
Sometimes, after publishing a post that mentions carryover cooking, I get questions in my email or on the post itself about why we don’t publish a carryover cooking table. A close look at the factors above should help to answer that question. With so many variables in a cook, publishing a chart that shows carryover times would be practically impossible. We’d need an entry for every cook temp, for every mass, for every shape, and for every kind of meat or bread. That doesn’t mean you should give up hope of mastering your cook. It just means you’ll have to get a feel for how much carryover to expect given the specific circumstances of each cook.
Using tools such as the ChefAlarm®, with its Min- and Max-temp display can help you establish the carryover for any given recipe. Use it on a roast chicken to find out how much the meat cooks after you take it out of your oven, and then adjust your pull temp accordingly. Write the proper pull temp in your recipe so you can get it right again next time. With a little practice, you’ll be nailing temperatures each and every time you cook.
Conclusion
When cooking, temperature matters. Using a thermometer to gauge your pull temps is in every way better than using physical artifacts to check doneness. And if pressing a steak or cutting a chicken is the wrong way to hit an exact temperature, how much worse are they for determining a temperature you have not yet arrived at?
If you want to perfect your dish, you have to account for carryover cooking, and the only way to do that is to use accurate, reliable temperature instruments. Yes, there is a learning curve as you account for cook temp, the size of your food, and the kind of food you are cooking. But as you learn how those variables operate in your own kitchen, you’ll start to elevate your cooking in ways you’ve never imagined.
Shop now for products used in this post:
ThermaQ Blue | ChefAlarm | Thermapen |
|---|


very interesting data. especially since the salmon carried 20 degrees. id be curious to see some data on burger carry over for various sizes, cooking methods (grill, cast iron burger etc). Because ive been noticed some huge rest times on burgers out of a cast iron.
But i am curious, when you pulled the items from the heat, they remained on the hot tray correct? how would this experiment have differed if they were taken off the hot tray while resting?
Kane,
Actually, when I removed them from the oven, I put them onto a room-temp tray. If you were to leave them on the hot tray, the carryover would almost certainly be greater.
Very good. As an Engineer, I love the science of thermodynamics. It so relevant to everything.
One of my takes on a possible feature : I think we could determine the thermal constance by watching step response. Then Predict time before reaching pull temperature. Done by the controller / temperature monitoring device. However, my experience show some weird stuff too. Like jumps and falls in temperature during the cook. Kind of like I’m watching collagen breaking down through the thermal response. Or it could be my probes cables are seeing condensation, causing reading variations. As the TC wires see varying resistance between them because of the moisture in the smoker. My device is NOT one of yours. But, uses type K TC’s.
Stan,
It would be interesting to hook up some kind of PID to a roast and see how well it predicts a pull temp for a desired doneness temp.
As for the strange rise/fall sequences, it is more likely that you are watching collagen melt than that the probe is being affected. The hot and cold junctions of the TC probe are not affected by the moisture of the cook.
Thanks for reading!
Excellent article. Thank you.
Way over my head to understand. Make it simpler!
Jim,
In essence, higher cooking temperatures will have more carryover and larger pieces of meat will also have more carryover. So a very large piece of meat cooked at a high temp will have lots of carryover while a small cut, cooked, low, will have very little. A large cut cooked low will have more carryover than a small one at the same temp. Planning for carryover cooking requires real thermal knowledge, and you get that from a thermometer, not from guessing or pressing on your palm.
For another explanation of this concept, take a look at this part about carryover in our turkey post.
Great article! I had no idea that carryover temps could be so much in a beef roast.
Thank you.
There are still more variables that confound this science for the everyday cook, particularly for roasts in the oven. The larger roasting pans can be like heat shields slowing the cooking process and protecting the meat from cooking on the bottom surface faster than with the sheet pans you show. Roasts are often placed on stands in the roasting pans, lifting them off the broad hot surface of the pan as well. Also which level the rack is located matters, with lower positions cooking the bottom of the roast first and faster. Lastly, the use of convection throws everything off, cooking the meat faster and perhaps more evenly all around the surface of a roast on a rack. I end up using convection for large roasts at a lower temperature and on a rack for evenness of cooking with a probe. There is an art and science to this all in the end, as well as knowing your oven and pans and techniques.
Joe,
Yes! There are SO many variables!
Wow! Great article, Martin! Thank you for taking a professorial approach to heat and the carryover impact of different temperature distribution situations in meat. Very helpful. Answered questions I’ve pondered. Will improve my end result. Well done!
Great article. I love the data (table and graph). Please keep this type of cooking information coming.
This was an excellent article- well written and informative. More such articles addressing the science of cooking are needed.
I really appreciate these articles!
I have learned a lot from reading these.
And, the topics and depth covered I have not seen anywhere else!
What container do you recommend for the resting phase after the meat is removed from the heat? What about the temperature of this surface? What exactly did you use in this experiment and was it covered or uncovered? I’m imagining the thermal conductivity of this tray would affect the outcome as well?
Tristan,
The thermal conductivity of the tray will technically affect the outcome, but we eliminated that as a variable by using the same tray for every piece of meat. Metal will pull heat away faster than ceramic, but a ceramic tray will have more heat capacity than a thin sheet tray. The carryover numbers that we saw were with meats places on a metal cookie sheet that was left open to the air. Covering could increase carryover by slowing the cooling of the exterior, but there are more factors that can go into that than I have time to test.
Ultimately, I think the thermal difference in resting-dish material will be negligible.
Thanks for the details Martin. I’m still working on getting this right so we’ll see how the next run tastes! Also I’m super happy with my BlueDOT and have learned a lot so far using it.
I purchased a Thermapen about one year ago and am overall very pleased with the results that I have been getting by cooking to specific temperatures as opposed to relying on experience and guessing. However, I do have a question about carryover cooking. When I let my meat rest and continue to cook, upon on uncovering the meat plate, I find the meat swimming in juices. If the juices are on the plate, they are not “in” the meat. Does carryover cooking and letting the meat rest, produce a dryer meat as opposed to serving the meat immediately, or does cooking to the desired temperature excessively dry out the outer meat portions resulting in and overall dryer result?
Tom,
There will always be some juice loss. But if you cut into the meat while the fibers are still constricting (heating), you’ll squeeze more liquid out. By letting it rest, you will absorb some of the juices back into the meat fibers.
Great article. Please note, however, that Kelvins are not properly referred to in degrees. Not sure why you even mentioned Kelvins since in the next sentence you change to °C anyway, and as you know, the gradient is the same for each scale.
I’ve known for a long time that meat, regardless of type, continues to ‘cook’ after removing it from the heat. What I never knew was the amount of difference there is in the different types of meat (pork, beef, chicken, etc) or roughly how much. This was a worthwhile article to learn a bit more. Thanks for sharing the details of your experiments.
How much carryover will I get On a inch and a quarter Ribey cooked at 650F ? Looking for medium Doneness.
Steve,
At that temp, I’d expect quite a bit of carryover. I’d probably pull 10°F below your target. But again, use a ChefAlarm or some other way to track the temps along the way to learn how it will act given the way you cook it.
Best articleI’ve ever read on “carry over/resting” temps. Like you say so many factors including “what find temperature do you like to eat your food”. I serve my food piping hot. I have a many friends who like their food just above warm. More complexity often fixed by adding near boiling gravy…
Great article, Martin. I have a question though. When I cook a pork loin at a low temp 250 degrees is it better to crisp up the meat by starting meat at a high temp 450 degrees for a few minutes and then turn down to 200 or is it better to get closer to pull temp and then pump up the heat to get crust texture and not overcook?
Loren,
There are merits to each method, but I prefer searing at the end. Pull the loin about 10°F early while you get the heat up to 450°F then toss it back in for a few minutes.
Fantastic article. Literally the best I’ve read on carryover. Thank you.
I do have a question. Do rest times and methods affect carryover? For example, if I pull a 5lb beef tenderloin cooked at a low temp (225), wrap it in foil and towels and place it in a cooler, should I expect a LOT more carryover if held for say, an hour?
What if I had two 5 pounders wrapped separately in foil in the same cooler?
Oooh, this is an interesting question. Yes, the holding method will affect carryover. You will get more carryover if you wrap your meat in foil because you’ll be trapping the heat inside, not losing heat to surface evaporation. A well-insulated roast will carry over more than a poorly insulated one. Two in a cooler will warm the cooler more than one will, making a warmer environment, thus increasing carryover, but probably not bay that much. If you’re worried about too much carryover, let the roasts sit on the counter for a couple minutes before wrapping them and putting them in the cooler.
great article to satisfy the coot and scientist
thank you