Accuracy: How to Know If Your Thermometer is Accurate
One of the most basic attributes of any thermometer is its accuracy. Inaccurate thermometers lead to misinformed decisions about cooking, health, manufacturing, or any other process involving critical temperatures and can lead to disastrous results. Small unobserved increases or decreases in temperature can actually have profound effects upon the growth of bacteria, the pliability of plastics, the interaction of chemicals, the safety of food, or the tenderness of meat.
And yet, unlike distance or volume, for example, that can be measured directly by a tape measure or a measuring cup, temperature is a measure of the average kinetic energy of the molecules in an object and cannot be measured directly. Instead, thermometers measure temperature indirectly, by actually measuring other physical phenomena that change as temperature changes. Things like…

- the expansion or contraction of liquids in the case of mercury or alcohol thermometers
- the expansion or contraction of metals in the case of dial thermometers
- changes in the speed of the flow of electrons in the case of thermistor thermometers, like ThermoPop®
- changes in the amplitude of voltage in the case of thermocouple thermometers, like Thermapen® ONE
More importantly, although we are interested in the temperature of the article or process we are measuring, the thermometer is always measuring the temperature of itself, not the temperature of the surroundings. This temperature represents the temperature of the surrounds if the thermometer is in thermal equilibrium with the surroundings. For example, when people use an old candy thermometer to measure the candy stage of boiled sugar/water mixture, the thing being measured by the thermometer is the expansion of the red-died alcohol in the glass tube after the bulb of the tube is placed in the boiling sugar mixture, not the expansion of the sugar mixture itself.
Thermistor thermometers measure the increase or decrease in resistance in the metal oxide semiconductor sheathed in their own probe tip. Thermocouple thermometers measure the increase or decrease in voltage in the alloyed metals inside their own probes.
In this way, thermometers are doubly removed from directly measuring temperature…
- unlike tape measures or measuring cups, thermometers don’t measure temperature directly, they measure other physical phenomena that change when temperature changes
- and thermometers don’t actually measure those physical changes in the material being measured, they measure them inside their own systems when exposed to the material being measured.

Because thermometers are not measuring temperature directly in this way, determining their accuracy and tracking their continued performance over time present unique challenges.
The markings on your tape measure or measuring cup don’t change over time, but thermometers can actually lose their accuracy over time. This potential for instruments to lose accuracy over time is sometimes called “drift,” because the relationship between the system of the thermometer and the actual properties of the material being measured can “drift” away from each other. Drift in thermometers necessitates periodic re-checks or “calibration.”
Read more about what affects the accuracy of thermometers below…
So How Can You Know that Your Thermometer Is Accurate?
There are two ways to know if your thermometer is accurate:
- test its accuracy yourself at a known naturally occurring phase-change temperature or
- for professional use, compare it to an established standard and certify its accuracy
Performing Your Own Accuracy Test
The easiest way to know that your thermometer is accurate is to perform your own test at home or at work at a known, naturally occurring phase-change temperature.
Normally, as energy is added to a material, its temperature goes up. But there are certain key temperatures at which the increase in temperature stalls for a time because the increase in energy in a material is driving a phase change. As water, for example, moves from its solid phase (ice) to its liquid phase, the temperature of the water stays constant at 32°F or 0°C. And, again, as water changes from its liquid phase to its gas phase, the temperature of the water stays constant at 212°F or 100°C at sea level as shown in the diagram below…
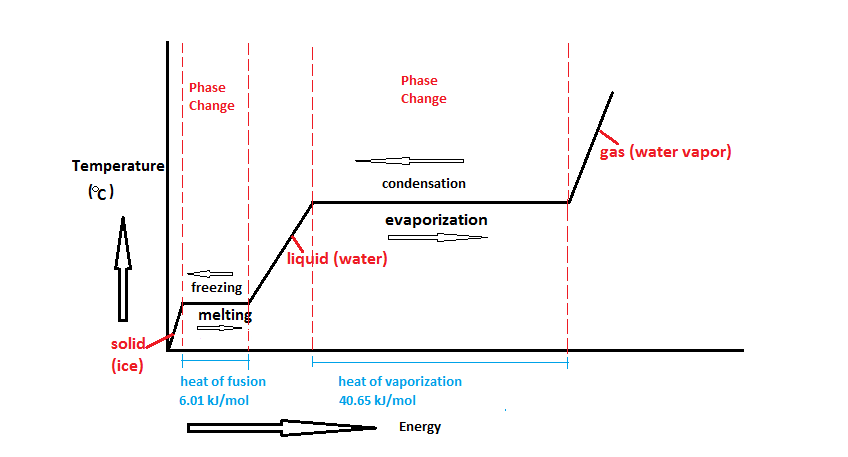

This fundamental feature of our physical universe makes it possible to be sure of the temperature of a material such as a properly made ice bath, for example, before you measure it so you can test your thermometer’s accuracy against that known temperature. To do this, you must carefully follow all the steps to create the ice bath and then note how far off from 32°F (or 0°C) your thermometer is reading. A thermometer that is reading within the published accuracy specification does not need adjustment or calibration. (You can, of course, use the boiling point to test the accuracy of your thermometers, as well, but you must know your local boiling point adjusted for elevation.)
A thermometer that is reading accurately at the ice point (or boiling point) will read accurately or within specification through its full range.

Some people wonder why they shouldn’t do such a thermometer speed test in food, since that is where they actually use their thermometers. The answer is that, under most circumstances, different places in the same foods are all at radically different temperatures. And those temperatures are actually changing while the food cooks. You can’t test the response time of an instrument getting to a target temperature if you don’t know what the target is. That is why the American Society for Testing and Materials (ASTM), the official body charged with developing and publishing technical standards for the assessment of instrument performance, recommends testing thermometer accuracy and response time in a properly made ice bath.
Established Standards
Professional businesses and organizations, in food service, for example, are often required to certify annually that their instruments are accurate. Comparing thermometers to established standards is the work of calibration laboratories, including the A2LA-Accredited Calibration Laboratory at ThermoWorks Headquarters in American Fork, Utah. Cal-lab accreditation, certification, and maintenance involve scores of tests and procedures for both the equipment and the staff. Those requirements are beyond the scope of this article, but suffice it to say that the American Association for Laboratory Accreditation (A2LA) regularly verifies our lab’s ability to operate according to international standards (ISO 17025) and satisfy the criteria we advertise in our scope of accreditation. And that in order to guarantee the accuracy of any given thermometer, its performance needs to be compared to another more accurate thermometer that is “traceable” to authoritative standards maintained by the federal government. Here in the United States, that means the National Institute of Standards and Technology, or NIST. Thermometers that are certified in such a way receive what is called a “NIST-traceable” calibration certificate and are known to be accurate.
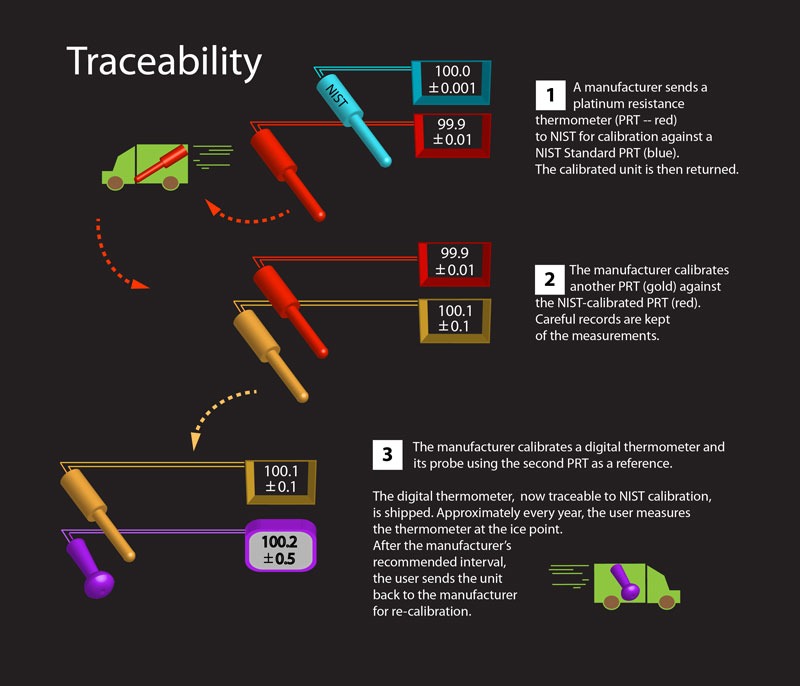
“Traceability” is characterized by several essential elements, including:
- An unbroken chain of comparisons going back to stated references acceptable to the parties, usually a national or international standard (i.e. NIST)
- Measurement uncertainty. The uncertainty of measurement for each step in the traceability chain must be calculated or estimated according to agreed methods and must be stated so that an overall uncertainty for the whole chain may be calculated or estimated. (This is represented as a plus sign over a minus sign in front of a number—e.g. ± 0.7°F.)
- Documentation. Each step in the chain must be performed according to documented and generally acknowledged procedures, and the results must be recorded.
- Competence. The laboratories or bodies performing one or more steps in the chain must supply evidence for their technical competence. This is the job of certifying entities like the A2LA, the American Association of Laboratory Accreditation.

Most ThermoWorks thermometers come with their own NIST-traceable calibration certificates verifying the accuracy of each individual unit, including Thermapen® ONE, Classic Thermapen, EXECTM, ChefAlarm®, DOT®, SPOTTM, and all the ThermaData® loggers.
Things NOT to Do When Testing the Accuracy of Your Thermometer
It is worth noting some common mistakes that people make when attempting to verify the accuracy of thermometers…

1764 °F) and is constructed from the purest grade of platinum scientifically available
- Do not compare your thermometer’s accuracy to that of another thermometer that is not traceable to an established national standard! This is literally the case of the blind leading the blind as you have no way of knowing that the other thermometer is accurate.
- Do not test your thermometer’s accuracy in food as you have no way of knowing what the temperature of the food is with certainty. Cooking food will have many different temperature gradients in it AND will be changing temperature while it cooks, so testing thermometer accuracy in cooking food is a particularly bad idea.
- Do not test your thermometer’s accuracy in an improperly made ice bath, like a glass of ice water as it will not be at the phase change point.
- Do not test your thermometer in boiling water unless you have done the work to determine what the actual boiling point is at your elevation. Boiling temperatures vary quite widely, unlike the ice point.
- Do not let your thermometer’s probe rest on the bottom, sides, or even the top of your properly made ice bath or boiling water. The bottom and sides of the vessel will be at a different temperature because of the vessel itself. And the top 1/2 inch or more of the ice bath will not be at 32°F (0°C) because of its interaction with the air. Instead, plunge the probe tip into the center of the ice bath and gently stir to avoid resting on individual ice cubes or boiling bubbles.
What to Do If You Determine That Your Thermometer Is Out of Spec
All quality thermometers come with published accuracy specifications. For example, Thermapen ONE has an industry-leading accuracy spec of ±0.5°F (±0.3°C) whereas ThermoPop 2, our top-rated inexpensive instant-read thermometer, has an accuracy spec of ±1°F (±0.5°C) over much of its range.
The published accuracy specifications should be listed on the box or in the instructional material of your thermometer. If you’ve thrown those materials away, you should still be able to find the accuracy specs for your thermometer by searching its model number on the internet.
Thermometers that are reading within their published specification do not need adjustment. This is a critical point. For example, if your Thermapen ONE should read between 31.5 and 32.5°F and your ThermoPop 2 should read between 31 and 33°F in a properly made ice bath. Other manufacturers’ instruments should read within their published specifications, as well.
If an instrument is reading outside of its spec, it needs to be calibrated. Some instruments, including Thermapen ONE, have a trim calibration function that allows you to make adjustments to bring the instrument back into specification. Otherwise, you can send the instrument back to the manufacturer for calibration or repair or replace the instrument.

What Affects the Accuracy of Thermometers?

There are many things that can cause drift and affect the accuracy of a thermometer, depending on the type of thermometer being used, of course. But since all thermometers—except infrared thermometers—involve physical components that come in contact with the substance being measured, the most common culprits include…
Poor Quality Materials or Manufacturing
Poor quality raw materials or manufacturing processes can result in substandard instruments that cannot be trusted. Inferior brands that skimp on quality and construction will experience system latency, error, and other impacts compromising their accuracy. ThermoWorks only uses the finest quality metals, circuit boards, reinforced housings, etc. and exercises the utmost care in their assembly by hand with multiple test points along the way to ensure performance.
Physical Shocks
The metal springs in the probes of dial thermometers are notorious for getting out of alignment with almost every use and needing daily calibration. But the physical components of any type of thermometer can be jostled—drops, knocks in a drawer, or other physical shocks can change the physical orientation of the components, compromising their accuracy.
However, thermocouples, when assembled appropriately into probes are essentially immune to physical shock. And certain types of thermocouples, like the Type K thermocouples in your Thermapen, are known to maintain their accuracy more durably than thermistors and other types of sensors, despite knocks, jostling, or drops, even severe ones in most cases.
Thermal Shocks
Measuring an extreme temperature, either on the high end or the low end of a poor quality thermometer’s stated range can affect the ongoing accuracy of that thermometer and may require calibration. Sensors in higher quality thermometers are designed for a wider range of temperatures than stated in the spec table, allowing you more room for measuring without shocking the system. The Type K thermocouple used in the Thermapen ONE, for example, has capabilities for a much wider range than the stated specification, which reflects the design criteria, not physical limitations of the thermocouple itself. This feature ensures that Thermapen ONE’s thermocouple sensor will not drift prematurely as a result of use within the stated temperature range in the spec table.
It should also be noted that thermometers of all sensor types sometimes have stated specs detailing less precise readings at the far ends—hot and cold—of their stated ranges.
Circuitry
Electronic thermometers must perform both accurate electrical measurements and complex calculations to display a temperature result. In the case of thermocouple thermometers, the temperature of the cold junction must also be measured and factored into the calculation. Additionally, any connections between the temperature sensor (the probe) and the display (the meter) can introduce errors and reduce repeatability.
The Thermapen circuitry is designed with high quality components to reduce electrical measurement errors and a very high clock speed to manage the computations. Furthermore, by permanently joining the meter and probe, Thermapen removes any source of error attributed to connection issues.

Speed
Thermometers can only tell you what the temperature of a substance is once the sensor at the tip of the thermometer has reached thermal equilibrium with the substance being measured. In the case of poorly made thermometers or thermometers with particularly thick probe sheaths, this equilibration can take several seconds—even up to dozens of seconds. Users of slow thermometers typically do not wait long enough for their thermometers to take a “full reading” before moving on, so they make decisions based on incomplete—and thus inaccurate—measurements.
Conclusion
Temperature and its measurement has a huge impact on our quality of life, allowing us to not only plan appropriately for conditions, but to control them—whether it’s the temperature of the air around us, the temperature of our bodies, or the temperature of the food we cook. Our ability to determine and control temperatures is only as good as the accuracy of the instruments we use to measure temperature. And because thermometers have no way of directly measuring the temperature (or the average kinetic energy of the molecules) of any system, we are all also beholden to a complex system of standards and calibrations to verify the accuracy of our instruments.
How should knowing this impact your own use of thermometers?
- Pay attention to the sensor type of the thermometers you use and learn about their strengths and weaknesses
- Know the published accuracy specifications of the thermometers you use
- Know how to verify the accuracy of your thermometers, either through
- calibration and certification to a known standard
- or through the use of a properly made ice bath test
- Be prepared for lesser quality thermometers to “drift” over time (or become less accurate), while top quality thermometers, like Thermapen ONE, maintain their accuracy over years and decades (that’s one of the things you’re paying for)
Being an informed thermometer user and consumer will enhance your ability to make good decisions about cooking food, travel, and everything else you do in life.







Does an ice bath work to verify the accuracy of an air probe?
Yes! but it’s response time will be slower, on purpose. You’ll need to suspend it in the ice for longer to get an accurate reading.
Very informative
Thank you for educating me in the science of measuring temperatures of quality manufactured instruments!
Did I miss it? How do I make a “properly made ice bath”?
Follow the steps outlined in this post: How to Properly Make an Ice Bath
It says to make sure to use a properly made ice bath… But you (the Article) does not say how to make that ice bath.!
An oversight, indeed! You can get the instructions here: https://blog.thermoworks.com/thermal-secrets-creating-properly-made-ice-bath/
Is home calibration possible on a Chef Alarm? If so, what is the calibration process?
Test it using a properly made ice bath. If it doesn’t come in on spec, call our in-house tech support to find out what’s wrong or what can be done about it.
Thank you for going the extra mile to write this piece. So much attention to detail. It’s not only informative, but also really well written.
Thanks!
Thanks, a very informative white paper regarding the ins and outs of temperature calibrations. I look forward to my new RFX instrument.
My thermapen won’t turn on. I have changed the battery with no effect.
Any recomendations?
Contact our customer care and tech support! 1-800-393-6434.
“temperature is a measure of the average kinetic energy of the molecules in an object and cannot be measured directly. Instead, thermometers measure temperature indirectly, by actually measuring other physical phenomena that change as temperature changes.”
In a LiG thermometer, the height of the indicating liquid changes with density, which varies with the mean kinetic energy of the atoms (Hg) or molecules (ethanol) of the liquid.
density ρ = m/V
mean kinetic energy K = 1/2mv²
Isolating m and equating: ρV = 2K/v²
ρ=2K/Vv²
Density of a liquid is a direct measure of the molecular mean kinetic energy.
∆ρ=m/∆V; ∆V=m/∆ρ
The reading on a LiG thermometer, determined by ∆V = m/∆ρ, is a direct indication of ∆ρ and therefore a direct indicator of ∆K.
An exactly analogous density argument can be made for a bimetal strip thermometer.
Temperature is thus read directly, not by measuring other phenomena.
The other phenomena do not change with temperature. They change with kinetic energy.
Temperature is a direct indicator of the kinetic energy of the thermometric material. This will also be true of a PRT or a thermistor.
Measured temperature is determined by the thermometric scale on a thermometer. Therefore, temperature is defined as the reading of a thermometer.
Thermometers measure temperature directly. They measure K indirectly.
If you want to split hairs in that way, that’s fine. But it’s dependent on a definition of temperature as being “that which a thermometer measures,” which is not a very good definition. I have consulted with our Chief Metrologist, who was the former Metrology at Fluke, and he even showed me a whole BBC documentary about the quest for direct temperature measurement. But beyond that, everything you’ve described there is literally measuring a secondary phenomenon. Yes, every one of them has a direct correlation with the kinetic energy of the molecules. Yes, density is bound directly with temperature, but the expansion of alcohol or mercury or a bi-metallic strip IS a secondary measurement. We have found ways to cleverly correlate those things with the desired quanta we seek to measure, but a measurement of density is a measurement of density, not a direct measurement of kinetic energy, which is what temperature is.