Heat vs. Temperature: What’s the Difference?
Temperature and heat are not the same thing. I know that’s strange to hear, and it may seem counterintuitive. Confusion on this point is understandable; the difference may not seem important outside of a science lab, but understanding it better can help you understand and control the thermal processes that happen in your food every day. In this post, we hope to clear up what the difference is between these two thermal principles. Read on to learn more!
Temperature measures molecular speeds, heat measures thermal energy.
What is temperature?
While we tend to think of temperature simply as “how hot something is,” that’s not a very strict definition. In the terms of physics, temperature is a measure of the average speed of the molecules in a given piece of matter.
Every molecule of matter is always moving. They can be moving around freely—bumping into other molecules, as in gases and liquids—or just vibrating, wiggling in place while locked in a solid. The faster these molecules move, the higher the substance’s temperature. Take as an extreme example the difference between liquid water and solid ice: in the liquid form, water molecules move freely, at a higher average speed than the molecules in ice, stuck in their lattice. If the molecules move even faster, they break out of the water and turn into a gas: steam.
How temperature is taken
So how do we measure temperature? It’s a more interesting question than it may seem at first blush. We can’t just point a radar gun at a surface and read the speed of the molecules. (No, that’s not what an infrared thermometer does.) After all, at any time, half the molecules may be wiggling away from the radar and half may be wiggling towards it, which would (watch out for the math jargon!) yield a vector sum of zero.
To measure the temperature of an object or substance, then, we use known behaviors of a known substance to tell us what the temperature of the first object is.
In thermodynamics, there is a law (called the zeroth law) that says if A is at the same temperature as B and B is at the same temperature as C, then A is at the same temperature as C. This may seem super obvious, but it is strangely important to the foundation of this sturdiest of sciences. So if my water bath is at the same temperature as my probe tip, and my probe tip is at the same temperature as my thermal sensor, then my thermal sensor is at the same temperature as my water bath.
In the case of a mercury thermometer, the mercury comes to the same temperature as the water; in a bimetallic strip thermometer, the strip equalizes in temperature with, say, the roast. And in the case of Thermapen® ONE, the thermocouple-weld reaches the same temperature as whatever it is that we’re measuring—and it does it fast.
In each of these cases, we already understand how mercury acts, how a bimetallic strip acts, and how a thermocouple acts at various temperatures, so we create a system for displaying those properties in ways that show us the temperature.
What is heat?
Heat, on the other hand, is not a measure of the speed of molecules, but a measure of how much work can be done by the thermal energy in a system. Yes, heat is measured in the exact same units as other forms of energy—joules, calories, BTUs, etc.
‘Work’ can be interpreted in a number of ways here.
In the normal conception of it, “work” means moving things, and in truth, all the other definitions of it mean pretty much the same thing. When we shovel dirt in our gardens, we convert stored sugar and fat in our bodies into other chemical energies that move our muscles (we feel the inefficiency of our anatomical engines when the excess heat builds up in our bodies and we start to sweat to cool off). When heat is used to create electricity, it moves electrons around in coils and wires. And when we heat something on a stove, we make the molecules move faster.
I know that seems odd. We don’t typically think of a hot block of iron as being able to accomplish any work, but hot blocks of iron is pretty much how the industrial revolution started—heat generates steam, steam builds pressure, and pressure moves wheels. The more heat something has, the more work it can perform. This equivalence was discovered by James Joule and first published in 1843.
Wherever mechanical force is expended, an exact equivalent of heat is always obtained.
– James Joule
How are heat and temperature related?
Of course, despite being different, the two concepts are not inextricable from each other. Every material has a definite heat capacity, which is measured in joules per kelvin—energy per degree. Something with a high heat capacity can hold a lot of thermal energy for every degree of temperature.
How on earth does that make any sense? Well, take a set of blocks, steel and wood.
At cool room temperature, the steel block will feel almost cold to the touch, while the wood block will feel barely cool. That is because a steel block at a given temperature can contain more thermal energy than the wood can, so the heat from your hand flows more readily into it than into the wood. Each degree of temperature difference between the steel block and your finger can hold more energy than the temperature difference between the wood block and your finger. (The same goes for hot steel vs. hot wood…there is more energy to pump into your finger and burn you.)
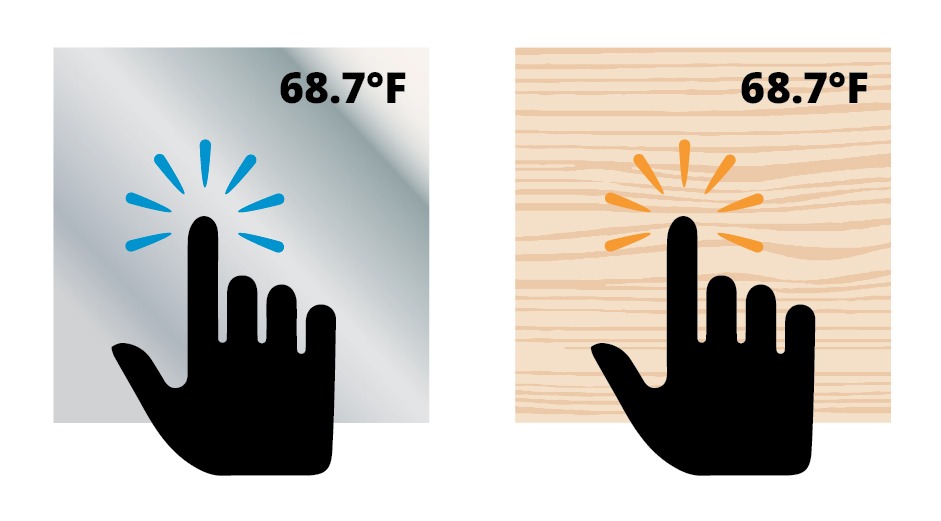
This is also why you can put your hand into a 350°F (177°C) oven for a minute, but you don’t even want to touch 350°F (177°C) oil!
What if we put the two blocks next to each other? Because they are at the same temperature, thermal equilibrium, the steel block will perform no direct heat work on the wooden block. Nor will it perform any work on another, smaller, steel block at the same temperature. Their thermal equilibrium prevents the flow of energy from one block to the other. There is no difference in their temperatures, so there is nowhere for the heat to flow.
(If we connect a heat pump to an object, we can harvest its heat energy to perform work elsewhere. One could conceivably attach a heat pump to one large block of steel, harness energy from it, and use that energy to run a motor to lift another, smaller, block of steel that is at the same temperature. So though it cannot perform direct, molecule-moving work on the smaller block, its heat could still be used to perform work on it.)
Heat transfer
It is also important to understand that there is no such “thing” as heat. Heat isn’t stuff, it’s not a substance—just like there is no such “thing” as cold. Cold is not something that battles against heat until there is a winner; rather, cold is just the absence of heat. The idea that heat is a substance has a long history. Scientists used to call it “the caloric.” But Joule’s discoveries helped put an end to that way of thinking.
When we warm something, it is not that we pump a hot substance into it; nor do we remove that substance when we cool things. When we say something “has heat in it” we are using shorthand for saying that something “has the thermal ability to perform work if the proper thermal-mechanical process is used to harvest and convert that energy.” Obviously, it is much faster and easier to say it has heat in it! When we heat something, yes it gets hotter, but it also receives more ability to get work done.
And heat only flows downhill. A cold block cannot get colder, donating its heat to a warmer block. A warmer block will always warm a cooler block. This is why and how carryover cooking works! the heat from the outside—the hottest—part of a chunk of meat flows thermally “downhill” into the cooler center.
Conclusion—heat in food
Cooking can broadly be considered as the application of heat to food. And, indeed, when we cook food, we perform physical and chemical work on it. the coagulation of proteins, the gelatization of starches, Maillard browning, caramelization—all of these are forms of work that heat from our oven or sautépan or pot of water performs on our food. And that’s why temperature matters, but different temperatures matter differently for different foods! A good thermometer like Thermapen ONE makes judging the temperature (and therefore energy content and doneness) of our food easy and fast. We hope this explanation has been clear and helps you to understand these vital thermal concepts. Now, take that knowledge and apply in the kitchen!


